الزمن عند المسلمين.. اعتمدوا خط غرينتش وأرّخوا أحداثهم بالساعات وصحابي يحدد وقت وصوله للمسجد النبوي بـ"الساعة الخامسة" | تراث | الجزيرة نت

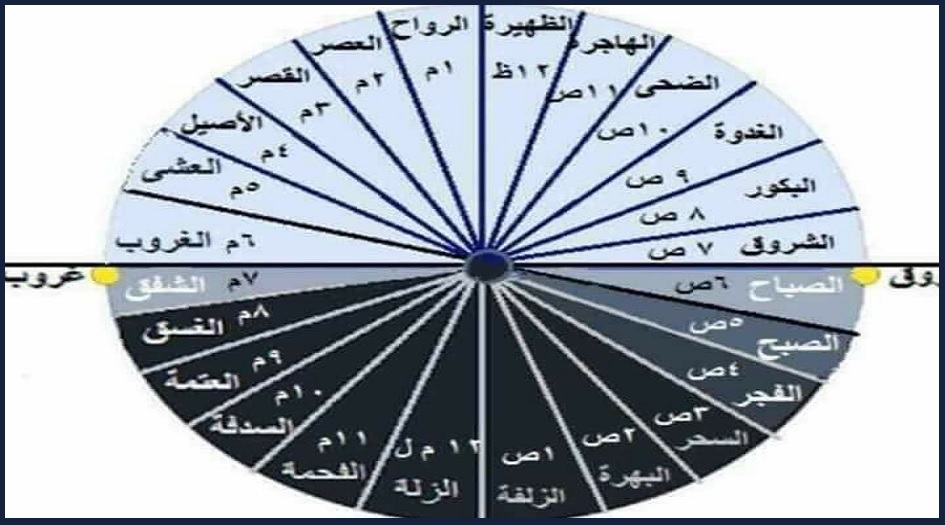
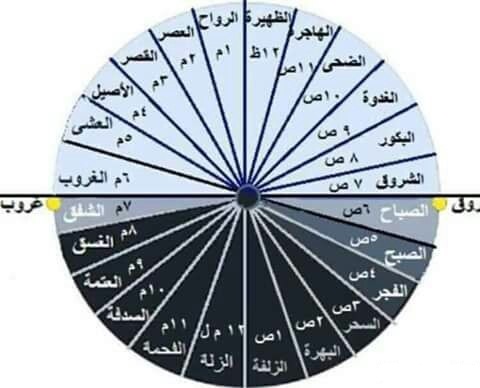
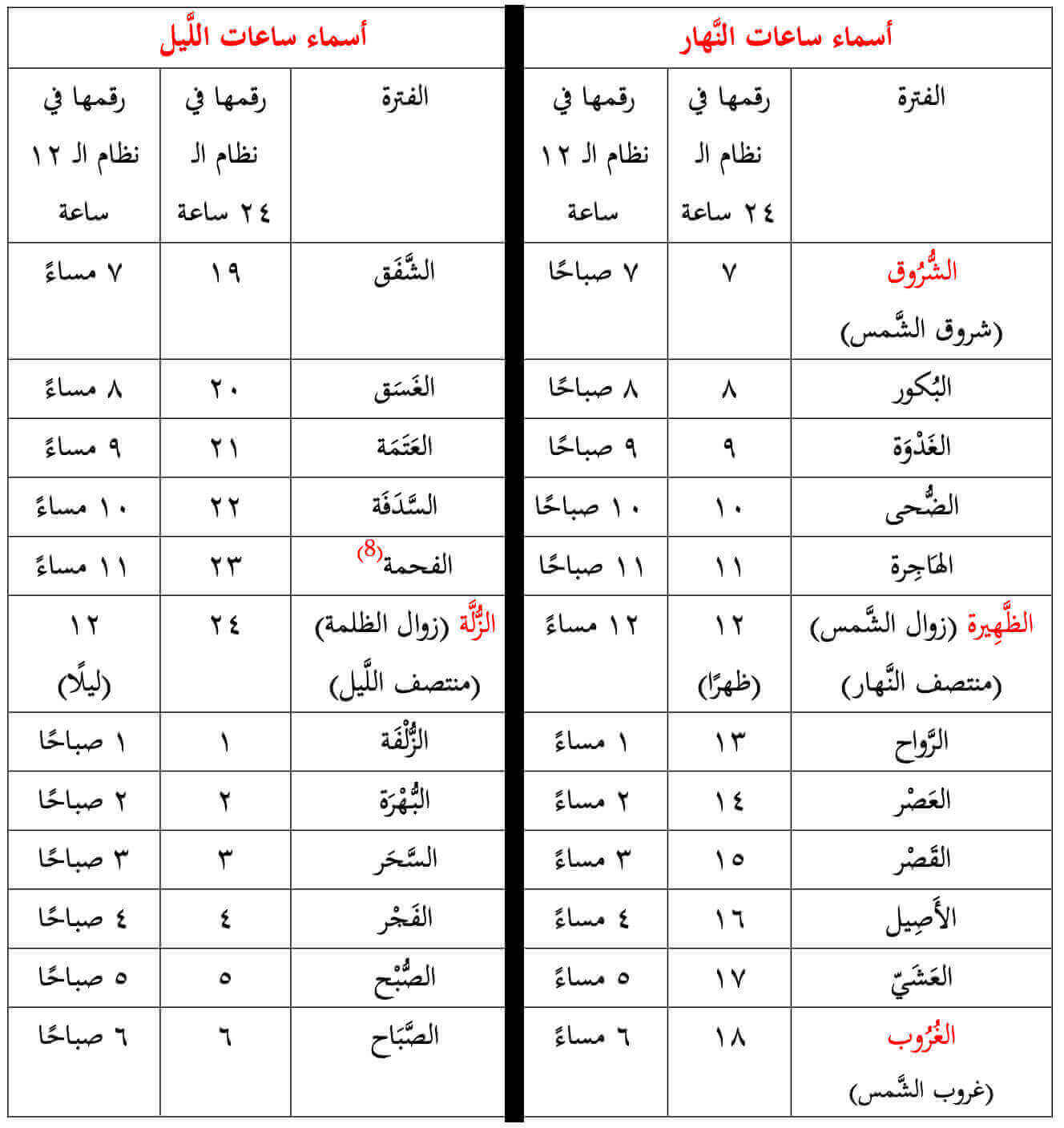
الباحثون المسلمون - إذا قال لك أحدهم بأن أذان العشاء سيكون في الساعة الواحدة وثلاثين دقيقة، فلا تسارع بالضحك، فليس بالضرورة أنه يمزح معك، بل قد يكون من أولئك القلائل الذين لا

روائع أدبــــــية☘ on X: "أسماء ساعات الليل والنهار عند الثعالبي وعددها 24. #نمير_ﺍلبيان #ومضة_حرف #أقصى_المحاني https://t.co/lSSjD1nkvq" / X